Top 10 Most Expensive Drugs in the World
Top 10 Most Expensive Drugs in the World
Updated on August 04, 2022 18:51 PM by Anna P
Before a drug can be prescribed to a person, research and clinical testing could sometimes extend for years, and so many drugs just don't work out. The entire study procedure can cease abruptly due to negative consequences and toxic reactions or economic factors.
The financial burden of research has a direct impact on the cost of therapy when the number of people affected by an illness is assessed in the thousands or even only hundreds internationally.
Medical drugs are a significant industry, with some selling for even several hundred thousand dollars annually. So, here we have compiled the list of the top 10 most expensive drugs in the world.
Spinraza (API Nursinersen)
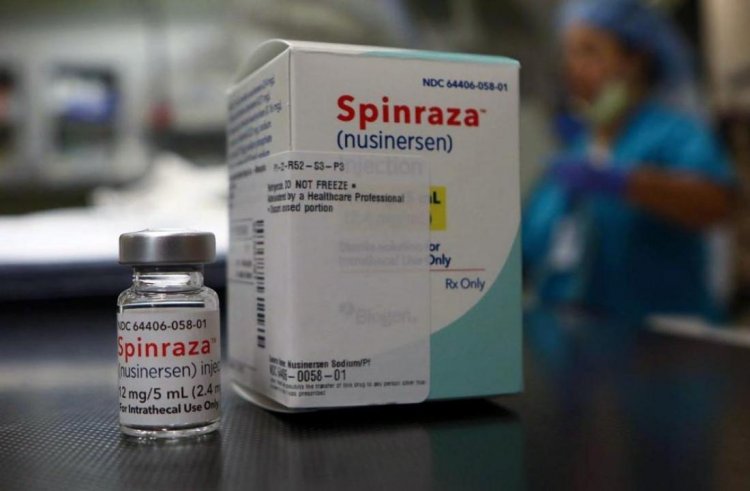
Spinal muscular atrophy is a condition that is treated with Nusinersen (marketed as Spinraza) (SMA). A mutation on the fifth chromosome (5q) causes SMA, which is characterized by progressive muscular atrophy that often starts soon after birth (although some forms of the disease have late-onset).
The medication is available as an injectable solution in 12mg vials. Specially skilled staff inject it directly into the spine. After the diagnosis, the first dose is given, and then one vial each at 2, 4, and 9 weeks later. Following then, a maintenance dose is given every four months for the duration of the patient's benefit.
Spinraza, a drug sold as Nusinersen, is used to treat the rare neuromuscular condition known as spinal muscular atrophy (SMA). It was the first medication for treating this illness to be licensed, and that was in December 2016. Nusinersen is classified as an "orphan medicine" in the US and the EU due to the rarity of the ailment it treats.
Add Block
Lunizyme (API Alglucosidase)
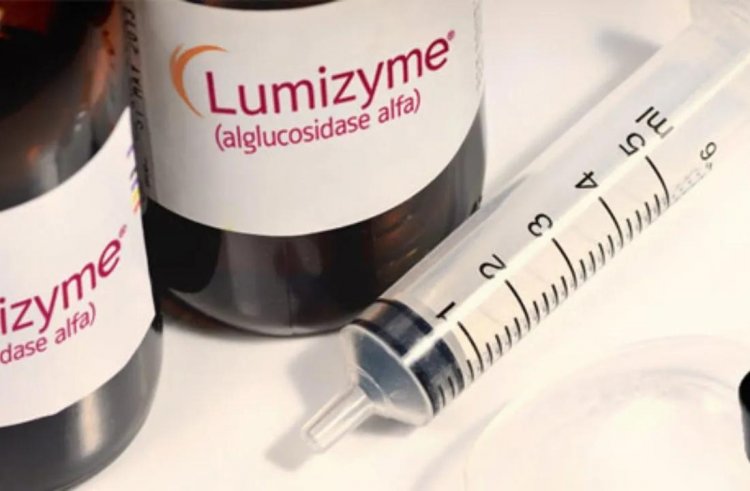
The medication is used to treat Pompe's illness (a rare, inherited disorder). Glycogen accumulates throughout the body as a result of the illness, which ultimately impairs the function of the affected organs (predominately muscles and liver).
In the US, one in every 40,000 people is affected. Every two weeks, a 20mg infusion of the medication is administered intravenously. The medication costs $870 for 50mg vials.
Enzyme replacement therapy uses Lumizyme (alglucosidase alfa). It takes the position of an enzyme (protein) that aids in the breakdown of glycogen, a substantial sugar in the body. Because either they lack this enzyme or it is inactive, people with Pompe illness are unable to break down glycogen.
Glycogen can therefore accumulate in the body and result in issues, particularly in the muscles. alglucosidase alfa, a substitute for the enzyme that enables the body to digest glycogen and utilize it as fuel, is called Lumizyme.
Elaprase (API Idursulfase)
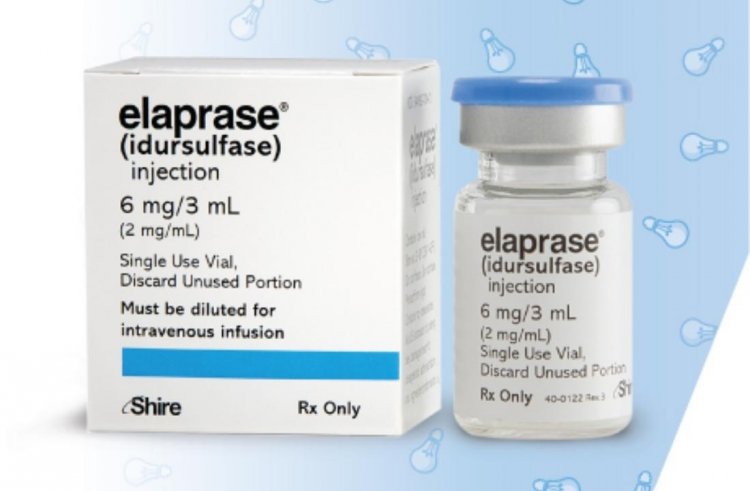
The medication is used to treat Hunter syndrome, a rare genetic condition that only affects males. Specific metabolic byproducts that can't be broken down accumulate in the body, making it difficult to walk and breathe. In the absence of treatment, the symptoms worsen with time. Every week, a gradual intravenous infusion of the medication is administered, with a dose based on the patient's body weight (0.5 mg/kg).
Add Block
Brineura (API Cerliponase alfa)
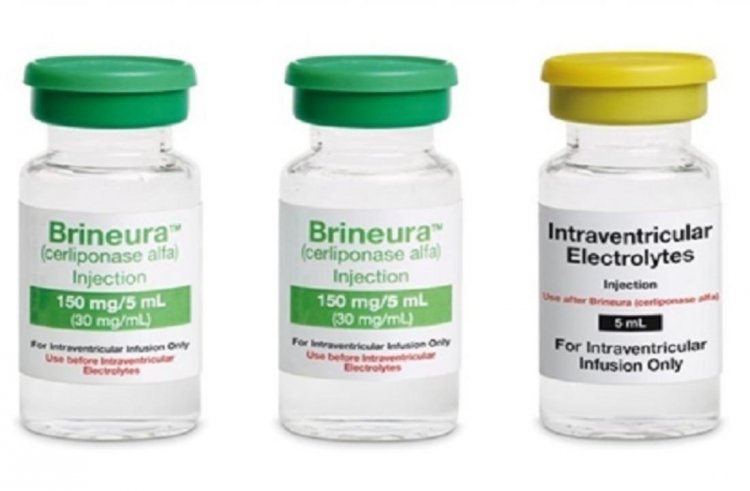
Neuronal ceroid lipofuscinosis type 2 disease, a rare condition that results in progressive brain damage, is treated with brineura. Every two weeks, the medication is administered through a specific tube that extends from the exterior of the skull to the site of infusion (inside the brain). As long as the patient experiences positive effects, the treatment continues.
Add Block
Soliris (API Eculizumzb)
A medication called eculizumab (marketed under the name Soliris) is used to treat a small number of disorders that damage red blood cells. It is a solution for intravenous infusion that is packaged in vials. The frequency of administration might range from once per week to once every three weeks depending on the patient's body mass, the stage of treatment (initial or maintenance), and the dosage.
The conditions paroxysmal nocturnal hemoglobinuria (PNH), atypical hemolytic uremic syndrome (aHUS), generalized myasthenia gravis, and neuromyelitis optica are all treated with the drug eculizumab, also known as the brand name Soliris.
It appears to not affect the risk of death in PNH patients, although it does lower the loss of red blood cells and the requirement for blood transfusions.
The first medication to be licensed for each of its uses was eculizumab, and this approval was based on small-scale studies. It is administered intravenously (IV) in a clinic.
It is only prescribed to those who have registered and adhere to a risk evaluation and mitigation approach, which involves advising people and making sure they are immunized. Side effects include a risk for meningococcal infections. It is a monoclonal antibody that has been altered to behave as a terminal complement inhibitor. It was created, produced, and sold by Alexion Pharmaceuticals, which held patent exclusivity up to 2017.
Carbaglu (API Carglumic acid)
Patients with excessive blood levels of ammonia are treated with carbaglu. There aren't many individuals who are suggested for this type of treatment because the indication region is limited to a few uncommon illnesses. The dosage per kilogram of body mass varies daily from 100 mg to 250 mg.
The cost of five 200 mg-active-substance tablets costs around $1,040, and the annual cost of treatment, which can reach $790,000 depending on the severity of the condition, is normally closer to half of that amount.
Carbaglu is a synthetic version of an enzyme found in the liver. This enzyme is required to handle extra nitrogen that is created when the body breaks down proteins. In the absence of this enzyme, nitrogen accumulates in the body as ammonia and is not expelled. Ammonia is extremely poisonous when it circulates in the blood and tissues and can result in death, coma, or permanent brain damage.
Hyperammonemia (HYE-per-AM-moe-NEE-mee-a), a disease of the urea cycle brought on by the absence of a specific liver enzyme, is treated with carbaglu. Carbaglu is typically administered along with other drugs to treat this chronic condition.
Add Block
Ravicti (API Glycerol Phenylbuutyrate)
Patients with abnormalities of the urea cycle are treated with the medication (UCDs). Patients with UCDs require a strict food regimen in addition to well-specified dosage and intake dynamics. Priced at $5,016 per vial, Ravicti is sold as an oral solution in 25mL vials. The dosage is determined by the patient's age and body surface area, so the annual cost of treatment might be up to $794,000.
The drug Ravicti, also known as glycerol phenylbutyrate (USAN), is used to treat a few inborn urea cycle diseases. The medicine functions by avoiding the body's toxic ammonia accumulation. It is a prescription medicine in the US that has FDA approval.
Anyone older than two months is permitted to use it. It was created by Hyperion Therapeutics based on the medication Buphenyl, which was already on the market, and it was approved on February 1, 2013. By imposing a high price on the medicine, Hyperion has come under fire.
The cost was fixed between $250,000 and $290,000. The drug's 2014 net sales of $30.8 million lagged well behind those of the more affordable and dated Buphenyl ($113.6 million in sales). Raciti was acquired by Horizon Pharma in March 2015 together with Hyperion Therapeutics.
Add Block
Luxturna (API Voretigene Neparvovec)

Adults and children with a particular type of retinal dystrophy are treated with luxury (mutations in gene RPE65). After a few days of preparation, the medication is administered as an intraocular injection (immunosuppressive medications lower the likelihood that the medication will be rejected by the patient's body). An expert eye surgeon administers the medication in a highly controlled hospital setting. The cost of the one-time procedure is $850,000.
A gene therapy drug called voretigene neparvovec, marketed under the trade name Luxturna, is used to treat Leber congenital amaurosis. Children's Hospital of Philadelphia and Spark Therapeutics collaborated in its development.
The US Food and Drug Administration has approved it as the first in vivo gene therapy (FDA). Biallelic RPE65-mediated inherited retinal disease, also known as Leber's congenital amaurosis, is a genetic condition that leads to progressive blindness.
The initial medication for this illness is Vortigern. Although the illness cannot be cured with gene therapy, people who receive it noticeably improved their vision. It is injected beneath the retina.
Glybera (API Alipogene Tiparvovec)
The gene therapy Glybera was created to treat the rare and fatal condition known as lipoprotein lipase deficiency. The procedure involved 60 intramuscular injections given in a single session (the therapeutic effect was intended to last for at least 10 years). Glybera, also known as the "million-dollar medicine," soon garnered notoriety among Europeans.
The maker took it off the market two years after receiving European permission. Only 31 patients had gotten the treatment as of 2018, and the product's maker (UniQure) no longer intends to market in North America. For pharmaceutical corporations, this medicine serves as a sobering reminder of how years of study can go in vain.
Gene therapy medication alipogene tiparvovec, marketed under the trade name Glybera, is used to treat lipoprotein lipase deficiency (LPLD), a rare genetic condition caused by mutations in LPL that can result in severe pancreatitis.
The European Medicines Agency recommended its approval in July 2012, and the European Commission followed suit in November of the same year. It was the first gene therapy treatment to receive marketing authorization in either Europe or the US. A single session of up to 60 injections into the leg muscles is used to deliver the medication. This treatment is meant to continue for at least ten years.
Zolgensma (API Onasemnogene abeparvovec-xioi)
The FDA approved a new form of gene therapy for SMA in May 2019, and at $2,125,000, it is the most costly medication ever created.
The medication, which is available as a suspension for intravenous infusion, is intended for use in children under the age of two who have SMA, even if they are not yet exhibiting symptoms (the diagnosis is made through genetic testing).
The only competitor on the market, Biogen's Spinraza, Zolgensma offers a definitive cure for the illness, which is the justification for the one-time therapy and the expensive price. Given the current clinical study data, it may eventually become the normative treatment for SMA.
ZOLGENSMA is made to replace the function of the survival motor neuron 1 (SMN1) gene, which is either absent or inactive, to target the genetic etiology of SMA with a single dose. The newly discovered gene instructs the survival of motor neuron (SMN) protein production in motor neuron cells. SMN protein is required for the survival and sustenance of motor neuron cells in muscles.





